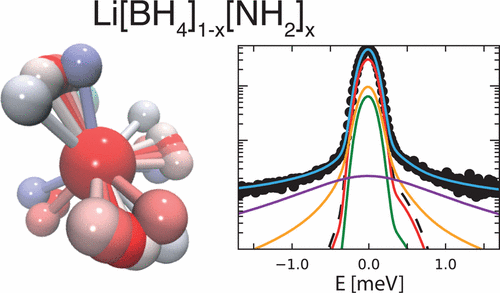
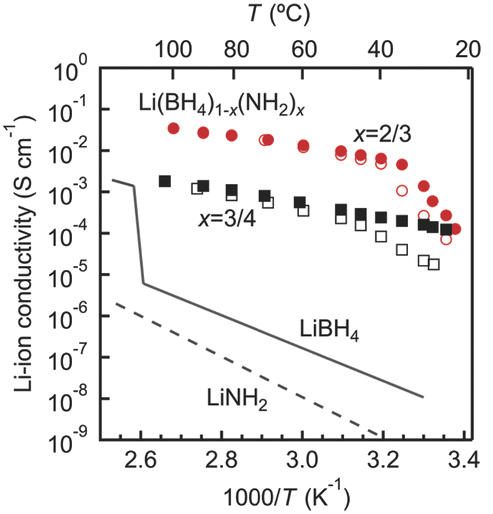
Lithium amide–borohydrides Li[BH4]1–x[NH2]x possess liquid-like Li superionic conductivity at nearly ambient temperature. The fast Li+ diffusion facilitated by the localized motions of the anions is proposed to occur through a network of vacant tetrahedral sites, acting as conduction channels. To study the reorientational dynamics of the anions, we have performed quasielastic neutron scattering experiments on samples with different compositions (x = 2/3, 0.722, 0.737, 3/4) over a broad temperature and time range. To unambiguously disentangle the contributions of the two species, [BH4]− and [NH2]−, we took advantage of deuterium labeling and could clearly demonstrate that the quasielastic broadening is mainly determined by the [BH4]− reorientations. With the help of a newly developed model, supported by ab initio molecular dynamics calculations, we have identified three relaxation components, which account for generally anisotropic C3-rotations of the [BH4]− tetrahedra including jumps by a small angle from the equilibrium position. |